Invasive tumors
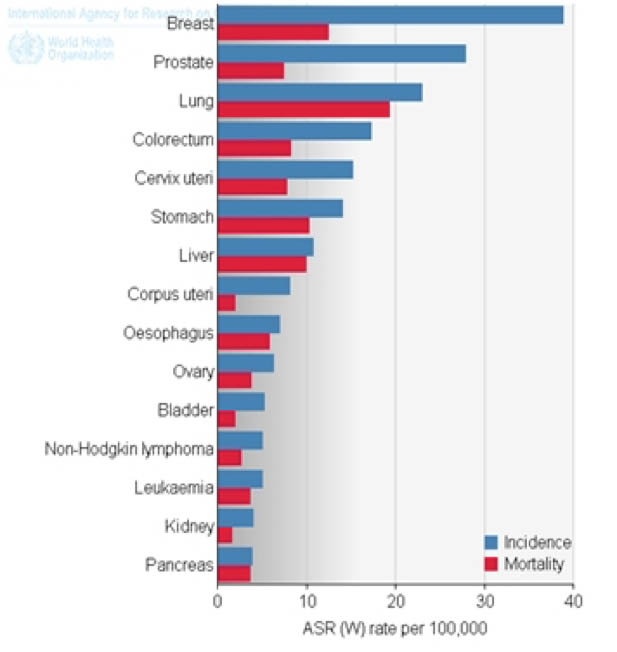
Cancer is one of the main publich healthcare problems. Invasive tumors develop beyond the organ where they originated, invading adjacent tissues and often distant tissues, leading to metastasis and high mortality rates.
In recent years, significant advances have been achieved in the diagnosis and treatment of early stages of cancer, when tumors can be surgically removed and treated, displaying a moderate mortality index.
However, very little progress has been made in the development of novel tools for the diagnosis and treatment of late and invasive cancer stages and cancer remains the main cause of death worldwide.
ONCOMATRYX scientists, in collaboration with prestigious academic and clinical institutions, have discovered a number of proteins in the tumor microenvironment, which are involved in tumor invasiveness, immune suppression and drug resistance. These stromal proteins represent novel biomarkers to develop accurate tools and methods for the diagnosis of invasive tumors.
COL11A1 biomarker in tumour invasiveness diagnostics
Many carcinomas present a desmoplastic reaction involving cellular and acellular components, such as fibroblasts, immune cells, blood vessels, the extracellular matrix, and soluble proteins such as cytokines and growth factors. This heterogeneous stroma seems to promote tumor growth, invasion, and resistance to chemotherapy. Fibroblasts in the vicinity of the tumor, the so called cancer-associated fibroblasts (CAF), play a role in stimulating tumor progression. However, researchers do not have specific tools to differentiate CAFs from inflammatory and resting fibroblasts.  Vimentin and alpha-smooth muscle actin are often used to identify CAFs, but these biomarkers are not specific, since they stain inflammatory fibroblasts and other cells as well.
Vimentin and alpha-smooth muscle actin are often used to identify CAFs, but these biomarkers are not specific, since they stain inflammatory fibroblasts and other cells as well.
One of the most significantly and consistently overexpressed genes in CAFs is collagen type XI α1 (COL11A1), a tumor-associated matrix collagen. COL11A1, like other collagens, is synthesized as procollagen by fibroblasts. Procollagens have a main central triple-helical domain, designated as α1, α2, and α3. Once secreted to the extracellular milieu, procollagens are cleaved and then the mature collagen molecules assemble extracellularly in fibrils. Collagen XI shares homology with other collagens, particularly with collagen V (75% homology at the amino acid sequence level). Transforming growth factor beta (TGF-β1) triggers the activation of smad2 signaling cascades, leading to transcription of COL11A1 and collagen remodeling. The specific expression of COL11A1 in cancer-associated fibroblasts might be an early indicator of tumor progression and infiltration.
Oncomatryx has successfully developed a monoclonal antibody which is highly specific for human proCOL11A1. It does not cross-react with other similar collagens such as COL5A1 and it clearly marks peritumoral stromal cells. Using this antibody, we have developed invaScan, an immunohistochemistry-based assay to detect proCOL11A1 in tumor biopsy samples.
