Diagnóstico del cáncer de vejiga
El cáncer de vejiga es el cuarto tumor maligno más frecuente en los varones y el noveno en mujeres. La mayor parte de los tumores vesicales son de estirpe epitelial siendo el 90 % de células transicionales, entre el 3-8 % son tumores escamosos y un 2-3 % adenocarcinomas. Desde las células epiteliales, el cáncer puede extenderse hacia las capas interiores de la vejiga alcanzando la capa muscular, y eventualmente invadir los órganos cercanos y nódulos linfáticos (metástasis). Los distintos tipos de tumores de vejiga podrían agruparse en tres categorías generales como in situ (Tis), superficiales (estadios Ta y T1) e invasivos (estadios T2 a T4). Los pacientes con tumores superficiales (75%) presentan recurrencias en un 70% de los casos en el curso de los años pero la progresión hacia la invasión del músculo ocurre en un 10-20 % de los casos. Los tumores de vejiga que presentan invasión del músculo en el momento del diagnóstico (20 % de los casos) tienen un pronóstico mucho menos favorable y a menudo progresan rápidamente.
El diagnóstico y la clasificación de un tumor de vejiga son cruciales para la aplicación del tratamiento adecuado. La consecuencia más inmediata de la estadificación es la operación a a la que el paciente será sometido para la resección del tumor. En el caso de los tumores invasivos, es necesaria una extirpación de la vejiga, con linfadenectomía y reconstrucción vesical, mientras que los tumores superficiales pueden ser eliminados mediante una resección transuretral, conservando la vejiga, y tratados con quimioterapio o inmunoterapia intravesical. Actualmente el diagnóstico definitivo de cáncer de vejiga se realiza mediante el análisis de tejido vesical extraido por cistoscopia. En este análisis puede determinarse también el estadio tumoral de manera inespecífica, ya que hasta el momento no existe ningún marcador molecular que permita objetivar la clasificación.
FGFR3
FGFR3 es un receptor de factores de crecimiento de fibroblastos, que posee un dominio intracelular proteina kinasa, y está implicado en la regulación de la proliferación, diferenciación y apoptosis celular.
Se han descrito varias mutaciones de FGFR3 en melanoma, cáncer de cerviz y mieloma múltiple. Estas mutaciones parecen activar el receptor, lo que deriva en un efecto oncogénico.
Aunque en los tumores de vejiga se han observado también algunas mutaciones del receptor, la alteración de FGFR3 más común en esta patología es la sobreexpresión de la proteína, tanto mutada como nativa.
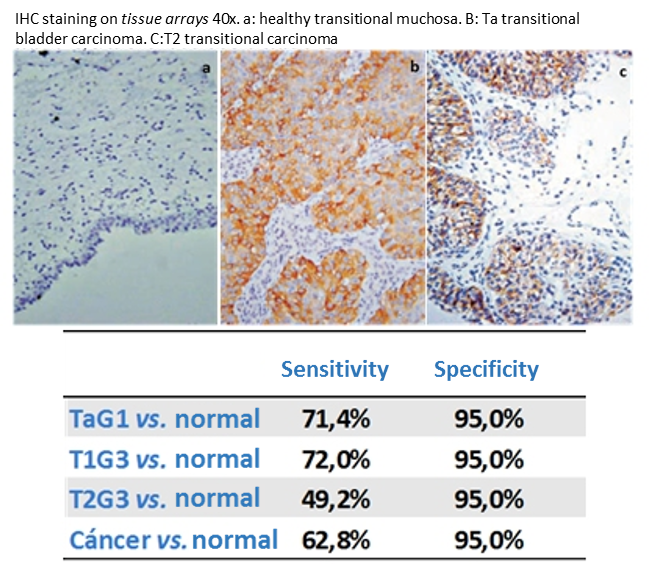
La sobreexpresión de FGFR3 en vejiga ocurre más frecuentemente en tumores superficiales , que no han invadido la lámina propria y son fácilmente resecables. Así, la detección de FGFR3 en los carcinomas vesicales, permite diferenciarlos del tejido sano, y determinar el estadio tumoral, distinguiendo los tumores en estadio Ta y T1, no invasivos y fácilmente resecables, de los T2, que requieren un tratamiento más agresivo.
FGFR3 se ha descrito además como diana terapéutica válida para el tratamiento de varios tipos de tumor, y de hecho son varios los fármacos antitumorales en desarrollo que se basan en la inhibición de este receptor para frenar el desarrollo tumoral.
DMTX bladderScan
DMTX bladderScan es un sistema desarrollado por ONCOMATRYX BIOPHARMA que permite detectar específicamente FGFR3 en muestras de tejido vesical.
Ventajas
Técnicas
- DMTX bladderScan se basa en un anticuerpo propio de Oncomatryx que detecta específicamente el
 dominio extrcelular de FGFR3
dominio extrcelular de FGFR3 - DMTX bladderScan no presenta reacción cruzada con otros FGFRs, tal y como se ha demostrado mediante inmunocitoquímica sobre distintas líneas celulares.
- DMTXbladderScan presenta mayor sensibilidad y capacidad cuantitativa que el anticuerpo comercial de uso más extendido
Clínicas
- DMTX bladderScan es apto para el diagnóstico del cáncer de vejiga en tejido mediante IHQ, así como para determinar la invasión del tumor, diferenciando estadios Ta y T1 de T2.
- DMTX bladderScan es especialmente sensible en la detección de carcinomas superficiales de vejiga, más difíciles de diagnosticar mediante las técnicas actualesLa detección de FGFR3 en tejido mediante
- DMTX bladderScan es útil para la selección de pacientes susceptibles de ser tratados con terapias antitumorales dirigidas a FGFR3
Bibliography
Fibroblast growth factor receptor 3 is overexpressed in urinary tract carcinomas and modulates the neoplastic cell growth. Gomez-Roman, J, Clin Cancer Res 2005;11:459-465
Targeting the extracellular domain of fibroblast growth factor receptor 3 with human single-chain Fv antibodies inhibits bladder carcinoma cell line proliferation. Martínez-Torrecuadrada et. al, Clin Can Res 2005; 11:6280-6290
FGFR3-targeted mAb therapy for bladder cancer and multiple myeloma. Hadari et al, J Clin Invest 2005; 119(5):1077-9
Frequency of fibroblast growth factor receptor 3 mutations in sporadic tumours. Sibley et al, Oncogene 2001; 20(32):4416-8
The incidence of thanatophoric dysplasia mutations in FGFR3 gene is higher in low-grade or superficial bladder carcinomas. Kimura et al, Cancer 2001; 92(10): 2555-61
The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Van Rhijn et al, Cancer Res 2001; 61(4):1265-8
Ectopic expression of fibroblast growth factor receptor 3 promotes myeloma cell proliferation and prevents apoptosis. Plowright EE et al, Blood 2000;95(3):992-8
Gly369Cys mutation in mouse FGFR3 causes achondroplasia by affecting both chondrogenesis and osteogenesis. Chen L. et al, J Clin Invest 1999;104(11):1517-25
¿TIENE UNA CONSULTA DE DIAGNÓSTICO?
Conozca las posibilidades de bladderScan
Please, fill this form
[contact-form-7 id=”361″ title=”YOUR DIAGNOSTICS REQUEST”]
Bladder cancer diagnostics
Bladder cancer is the fourth most frequent bladder cancer in men and the ninth in women. Patients with superficial tumors (75%) display recurrence in 70% of cases over the years, but progression toward muscle invasion happens in 10-20% of cases. Bladder tumors that display muscle invasion at the time of diagnosis (20%) have a much less favourable prognosis and often progress rapidly.
Diagnosis and classification of a bladder tumor is crucial for the application of the most suitable treatment. In the case of invasive tumors, the bladder must be removed, with lymphadenectomy and bladder reconstruction, whereas superficial tumors can be removed by means of a transurethral excision, preserving the bladder, and treated by means of chemotherapy or intravesical immunotherapy. The final bladder cancer diagnosis is currently made by means of the analysis of bladder tissue extracted by cistoscopy. In this analysis, the tumor stage can also be determined in a non-specific manner, as so far there are no molecular markets to facilitate the classification.
FGFR3 receptor
FGFR3 is a receptor of fibroblast growth factors, which has a protein kinase intracellular domain, and is involved in the regulation of cell proliferation, differentiation, and apoptosis.
Several FGFR3 mutations have been described in melanoma, cervix cancer, and multiple myeloma. These mutations seem to activate the receptor, which leads to an oncogenic effect. Even though some receptor mutations have also been found in bladder tumors, the most common FGFR3 disorder in this pathology is overexpression of mutated and native protein.
FGFR3 overexpression in the bladder happens most frequently in superficial tumors, which have not invaded the lamina propria and can be easily excised. Thus, detection of FGFR3 in bladder carcinomas makes it possible to differentiate them from healthy tissue and determine the tumor stage, distinguishing between Ta and T1 tumors, which are non-invasive and easy to excise, from T2 tumors, which require a more aggressive treatment.
FGFR3 has also been described as a valid therapeutic target for the treatment of several types of tumors. There are several drugs under development that are based on the inhibition of this receptor to halt tumor development.
DMTX bladderScan
DMTX bladderScan is an immunohistochemistry assay, based on a monoclonal antibody that specifically detects FGFR3 in tissue biopsy samples from patients with superficial bladder carcinomas.
Benefits
Technical
- DMTX bladderScan is based on an Oncomatryx proprietary monoclonal antibody that specifically detects the extracellular domain of FGFR3
- DMTX bladderScan antibody does not crossreact with other FGFRs, as it has been tested by immunocytochemistry on different cell lines
- DMTXbladderScan is more sensitive and more cuantitative than the most commonly used commercial antibody.
Clinical
- DMTX bladderScan is suitable for the diagnosis of bladder cancer in tissues by means of IHC, as well as to determine tumor invasiveness, differentiating Ta and T1 stages from invasive T2.
- DMTX bladderScan is particularly sensitive in the detection of superficial bladder carcinomas, which are harder to diagnose using current techniques.
- Detection of FGFR3 in tissue by means of DMTX bladderScan is useful to select patients who can be treated using anti-tumor therapies targeting FGFR3.
Bibliography
Targeting the extracellular domain of fibroblast growth factor receptor 3 with human single-chain Fv antibodies inhibits bladder carcinoma cell line proliferation. Martínez-Torrecuadrada JM. et al. Clinical Cancer Research 2005; 11(17):6280-90
Fibroblast growth factor receptor 3 is overexpressed in urinary tract carcinomas and modulates the neoplastic cell growth. Gómez-Román J. et al. Clinical Cancer Research 2005; 11:459-465
ANY DIAGNOSTICS REQUEST?
Make your question about Oncomatryx DMTX bladderScan
Please, fill this form
[contact-form-7 id=”361″ title=”YOUR DIAGNOSTICS REQUEST”]
Bladder cancer diagnostics
Bladder cancer is the fourth most frequent bladder cancer in men and the ninth in women. Patients with superficial tumors (75%) display recurrence in 70% of cases over the years, but progression toward muscle invasion happens in 10-20% of cases. Bladder tumors that display muscle invasion at the time of diagnosis (20%) have a much less favourable prognosis and often progress rapidly.
Diagnosis and classification of a bladder tumor is crucial for the application of the most suitable treatment. In the case of invasive tumors, the bladder must be removed, with lymphadenectomy and bladder reconstruction, whereas superficial tumors can be removed by means of a transurethral excision, preserving the bladder, and treated by means of chemotherapy or intravesical immunotherapy. The final bladder cancer diagnosis is currently made by means of the analysis of bladder tissue extracted by cistoscopy. In this analysis, the tumor stage can also be determined in a non-specific manner, as so far there are no molecular markets to facilitate the classification.
FGFR3 receptor
FGFR3 is a receptor of fibroblast growth factors, which has a protein kinase intracellular domain, and is involved in the regulation of cell proliferation, differentiation, and apoptosis.
Several FGFR3 mutations have been described in melanoma, cervix cancer, and multiple myeloma. These mutations seem to activate the receptor, which leads to an oncogenic effect. Even though some receptor mutations have also been found in bladder tumors, the most common FGFR3 disorder in this pathology is overexpression of mutated and native protein.
FGFR3 overexpression in the bladder happens most frequently in superficial tumors, which have not invaded the lamina propria and can be easily excised. Thus, detection of FGFR3 in bladder carcinomas makes it possible to differentiate them from healthy tissue and determine the tumor stage, distinguishing between Ta and T1 tumors, which are non-invasive and easy to excise, from T2 tumors, which require a more aggressive treatment.
FGFR3 has also been described as a valid therapeutic target for the treatment of several types of tumors. There are several drugs under development that are based on the inhibition of this receptor to halt tumor development.
DMTX bladderScan
DMTX bladderScan is an immunohistochemistry assay, based on a monoclonal antibody that specifically detects FGFR3 in tissue biopsy samples from patients with superficial bladder carcinomas.
Benefits
Technical
- DMTX bladderScan is based on an Oncomatryx proprietary monoclonal antibody that specifically detects the extracellular domain of FGFR3
- DMTX bladderScan antibody does not crossreact with other FGFRs, as it has been tested by immunocytochemistry on different cell lines
- DMTXbladderScan is more sensitive and more cuantitative than the most commonly used commercial antibody.
Clinical
- DMTX bladderScan is suitable for the diagnosis of bladder cancer in tissues by means of IHC, as well as to determine tumor invasiveness, differentiating Ta and T1 stages from invasive T2.
- DMTX bladderScan is particularly sensitive in the detection of superficial bladder carcinomas, which are harder to diagnose using current techniques.
- Detection of FGFR3 in tissue by means of DMTX bladderScan is useful to select patients who can be treated using anti-tumor therapies targeting FGFR3.
Bibliography
Targeting the extracellular domain of fibroblast growth factor receptor 3 with human single-chain Fv antibodies inhibits bladder carcinoma cell line proliferation. Martínez-Torrecuadrada JM. et al. Clinical Cancer Research 2005; 11(17):6280-90
Fibroblast growth factor receptor 3 is overexpressed in urinary tract carcinomas and modulates the neoplastic cell growth. Gómez-Román J. et al. Clinical Cancer Research 2005; 11:459-465